Our pharmaceuticals division is composed of a highly-skilled staff of experts working to select and develop state-of-the-art dossiers with the most excellent bioequivalence provided by cutting edge clinics. Our services include Due Diligence reviews, together with scientific, technical and legal revisions; we also provide translation services and we always work according to the latest European guidelines.
We provide full support for the development of new generic medicines: from the careful selection of the active principle to the lab development, until the final marketing and distribution of the new generic drug. No need to worry about learning all guidelines, current standards and official documentation and paperwork for the certification: our team is always up to date with every requirement needed and will take care of every issue for you.
Together with the development of generic pharmaceuticals, we are a key strategic partner to rely on for clients looking forward to creating a new pharmaceutical industry. We can provide plenty of expertise and know-how, together with infrastructures and connections.
(Click here to access the reserved area for the Generic Medicines division, to find the whole list of our products).
As a proof of our expertise and an evidence of the top quality of our activity, we have developed the nitroglycerin band aid in partnership with an Italian company, after years and years of research: we have introduced our “Nitro-patch” in the generic drugs market of Europe.
NITRO-PATCH
INTRODUCTION
In partnership with a cutting edge Italian pharmaceutical company we were the first to introduce a nitroglycerin band aid in the European market of generic medicines, after many years of pharmacological and pharmaceutical research approved by the American national regulatory body (FDA) and the European authorities. The technology of Nitro-patch is composed of:
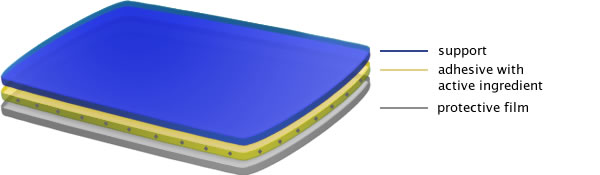
This band aid’s main feature is the fact that the active ingredient lies directly in the sticky part that comes in contact with the skin. The adhesive layer has a double function: on one hand, it attaches the item to the skin, and on the other hand it releases the active ingredient. A three-dimensional matrix calibrates the transfer speed of the active ingredient: 0.2 mg, 0.4 mg and 0.6 mg per hour for the related dosage – 5 mg, 10 mg, 15 mg. The nitroglycerin has proved to be satisfyingly absorbed: in fact, it can be detected in the plasma 30 minutes after the application of the band aid, while the maximum concentration is observed to be reached after about two hours. A constant plasma concentration can be registered within 24 hours, and is always proportioned to the selected dosage.
ACTIVE INGREDIENT
Nitroglycerin is used for the treatment and prophylaxis of angina pectoris, under strain or at rest, whether consequent to or associated with coronary insufficiency, as far as it provokes the vasodilation of the veins and a reduction of the resistance of the venous system (venous pooling), thus producing a consistent saving in cardiac activity and a reduction of the oxygen consumption by the myocardium. Transdermal system adapt perfectly to the use of lipophilic drugs acting at very low plasma levels – which is the case of nitroglycerin.
NITRO-PATCH
Nitro-patch is a little bio-engineering work of art as it can release the drug in the organism at a regulated quantity and speed for a set period of time, thus avoiding any drawback often caused by oral usage such as the loss of pharmacological substances caused by the interference of food and liver metabolism, as well as other risks connected with omissions or self regulation of the drug consumption that can occur because of the frequency of administration. Furthermore, the active ingredient directly seeps through blood for a prolonged period without any significant fluctuation, by that means avoiding a congestion of the organism and ensuring it is not left unprotected by the action of the medicine after just a few hours – something that may occur with common pills. Systemic action transdermal band aid technology is therefore a means for the constant administration of the drug. This enhances the reduction of potential side effects, and at the same time increases the tolerance of the cure among patients, as the active ingredient is gradually introduced into the circulatory system.
PARTNERS
Bouty is a top-quality company with proven experience in the pharmaceutical industry since the early 20th Century that has maximized its skills and developed new research methods in the field of drug delivery systems.
I.P. A.S., CRO, was founded in 1933 and is nowadays a well-known team of experts in Phase 1 and bioequivalence studies delivering about 40/45 studies per year, requested by more than fifty international sponsors: as for Nitro-patch, the company was in charge of proving all the pharmacological and pharmacokinetic features of the product mentioned above.
The Nitro-patch has been marketed in Spain by our partner Arafarma Group as the first branded generic transdermal band aid, with the name of Nitrofix ™. The product has also been distributed in Italy by several cutting edge companies leading the cardiovascular field.



